Influenza in the Americas causes significant deaths, especially in seniors and young children. Effective vaccines are crucial, but their composition must be updated annually due to viral changes. The REVELAC-i network, established by PAHO and CDC to evaluate influenza vaccine effectiveness across Latin America and the Caribbean, is an initiative that aims to improve public health decisions by generating data on vaccine performance and impact, ultimately reducing influenza morbidity and mortality. Learn about influenza prevention, vaccine effectiveness, and regional efforts to combat the virus.
Coronaviruses are a large family of viruses that can cause diseases in animals and humans. In humans, it is known that several coronaviruses cause respiratory infections with symptoms that range from the common cold to more severe diseases such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), identified in 2003 and 2012, respectively. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease (COVID-19) and was identified for the first time in Wuhan, China, in December 2019. By the end of March 2020, more than 400,000 COVID-19 cases were reported in over 150 countries. As of April 2021, more than 132 million cases and 2.9 million deaths had been reported throughout the world. In the Region of the Americas, more than 57 million cases and 1.4 million deaths have been reported since the onset of the pandemic. Brazil, Colombia, Argentina, and Mexico are the most affected countries [1].
COVID-19 immunization is considered an essential public health intervention for controlling the epidemic, together with other social and public health measures. COVID-19 vaccines can play a critical role in reducing mortality and severe morbidity due to COVID-19 and the spread of SARS-CoV-2. As of April 2021, more than 200 COVID-19 vaccine candidates are currently in the clinical development phase [2]. Of these, nine vaccines were recently authorized by regulatory agencies for use in Latin America and the Caribbean. A total of 49 countries and territories have initiated vaccination against COVID-19 in the Region [3]. Clinical trials conducted by the manufacturing laboratories have shown a favorable safety profile and high efficacy for these vaccines, ranging from 70 to 95% against cases of symptomatic disease.
Evaluation of vaccine effectiveness under operational conditions is essential for guiding decisions and evaluating the impact of the vaccination program as a whole. Vaccine effectiveness studies evaluate the protection that vaccines confer for different levels of disease severity and different intervals between doses, as well as to measure the effectiveness of incomplete vaccination and the duration of long-term protection. Furthermore, effectiveness evaluations help to understand the effectiveness of the vaccines in preventing infections, modifying the clinical course, producing less severe conditions, and reducing transmissibility.
For this purpose, a working group has been created for evaluating the effectiveness of COVID-19 vaccines based on the sentinel surveillance strategy for severe acute respiratory infections (SARI), using existing regional platforms such as the Severe Acute Respiratory Infections Network (SARInet) and the Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean - influenza (REVELAC-i).
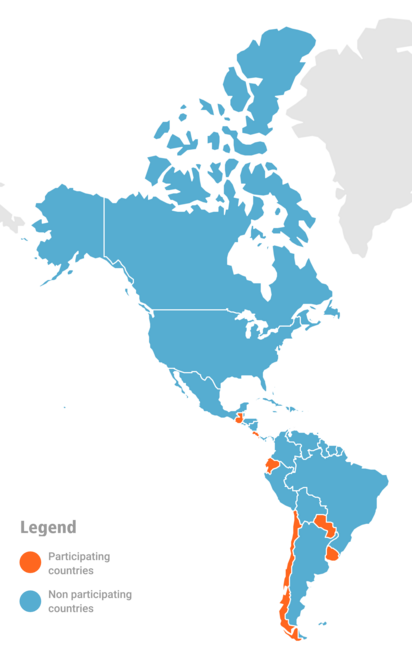
In 2020-2022, six countries: Chile, Costa Rica, Ecuador, Guatemala, Paraguay and Uruguay contributed their data to estimate the effectiveness of COVID-19 and influenza vaccines.